First-of-its-kind oral diabetes drug got approval in the US
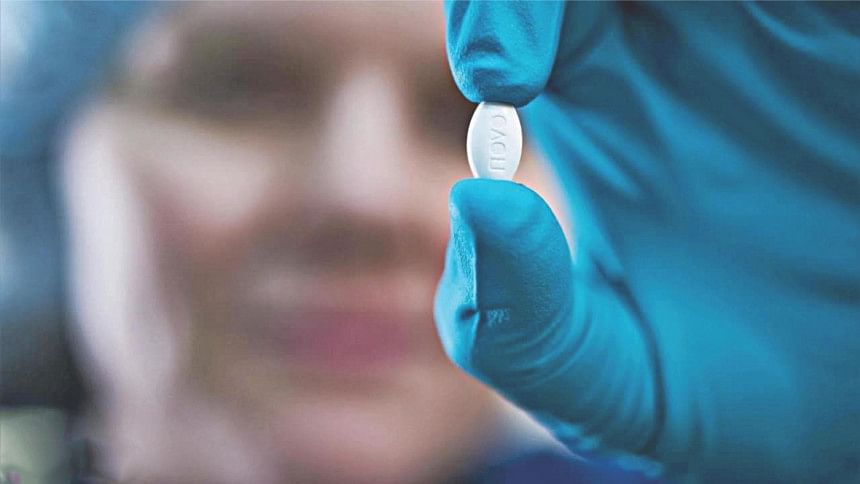
Novo Nordisk announced that the US Food and Drug Administration (FDA) has approved Rybelsus® (oral semaglutide tablets) on Friday September 20, 2019, as an adjunct to diet and exercise to improve glycaemic control in adults with type 2 diabetes mellitus.
The world's biggest producer of diabetes drugs already sells an injectable once-weekly version of semaglutide under the brand name Ozempic. But now it is hoped to transform the market by offering patients a non-injectable treatment for the disease. The new drug stimulates insulin production in patients with type 2 diabetes, the most common form of the disease, and is meant to be taken once a day.
Rybelsus®, the brand name for oral semaglutide in the US, is the first approved
analogue of the naturally occurring glucagon-like peptide-1 (GLP-1) receptor agonist in a tablet. It is administered once daily and is approved for use in two therapeutic dosages, 7 mg and 14 mg.
The approval of Rybelsus® is based on the results from 10 PIONEER clinical trials which included 9,543 adults with type 2 diabetes.
Rybelsus® more effectively lowered blood sugar than sitagliptin and empagliflozin. Furthermore, treatment with Rybelsus® resulted in up to 4.4 kg reduction in body weight. Rybelsus® demonstrated a safe and well-tolerated profile across the PIONEER programme, with the most common adverse event being mild to moderate nausea which diminished over time.
"We are very excited that we can make the first oral GLP-1 available in the US and thereby expand the treatment options for adults living with type 2 diabetes," said Mads Krogsgaard Thomsen, executive vice president and chief science officer of Novo Nordisk. "Novo Nordisk has a very long legacy of developing innovative injectable medicines for people living with diabetes and, with the approval of Rybelsus®, we are now able to bring our innovation into the market for oral antidiabetics."
Novo Nordisk plans to make Rybelsus® available to adults with type 2 diabetes in the US in the fourth quarter of 2019.
Rybelsus® is currently under review by several regulatory agencies, including the European Medicines Agency and the Japanese Pharmaceuticals and Medical Devices Agency.
The approval follows close on the heels of the European Association for the Study of Diabetes (EASD) annual meeting, which wrapped Friday in Barcelona. And there, the excitement over Rybelsus was palpable, Novo CSO Thomsen said.

 For all latest news, follow The Daily Star's Google News channel.
For all latest news, follow The Daily Star's Google News channel. 



Comments