Existing cold chain not enough for Covid-19 vaccines
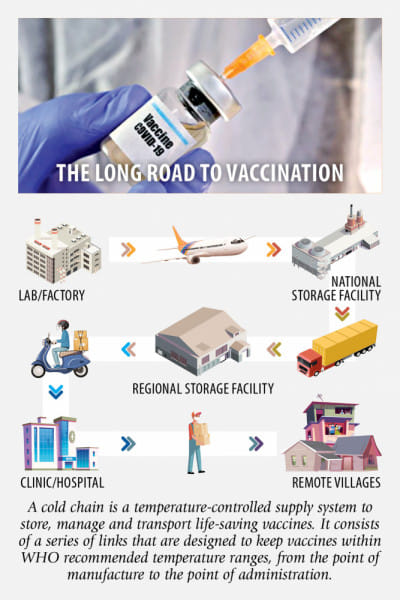
After the government recently signed a trilateral memorandum of understanding (MoU) with Serum Institute of India and Bangladesh's Beximco Pharmaceuticals Ltd to get three crore doses of Covid-19 vaccines from Serum—which will be collected and transported by Beximco to the storage facilities under the health directorate's EPI—the challenges Bangladesh would face in scientifically preserving and delivering the vaccines with its existing cold chain network have come under the spotlight. According to the deal, the three crore Covishield doses will be delivered in phases, with 50 lakh doses every month.
Although we have a very good network of EPI (Expanded Programme on Immunisation) which is capable of keeping life-saving drugs at temperatures as low as minus 20 degrees Celsius, it is basically used for vaccination of children and has a capacity of storing, transporting and distributing around 1.5 crore doses at a time, according to government officials. So experts believe, with the existing facilities, it will be really difficult to store and deliver such a large volume of Covid-19 vaccines within a short time since those will need to be kept within the WHO-recommended temperature ranges, from the point of manufacture to the point of administration. Sadly, for us, the main challenge will be to efficiently deliver the vaccines to the health centres across the country.
We understand that the government is thinking of purchasing vaccines that can be managed with its existing capacity, as upgrading the present system will be very expensive. As we know, different vaccines require different temperatures, cold chain facilities and handling procedures. While the vaccine being developed by Pfizer requires a storage temperature of minus 80 degrees Celsius, the one being developed by Moderna was initially stored at minus 70 degrees Celsius (although now the company plans to ship the shots at minus 20). And the Covishield vaccines that Bangladesh will purchase are supposed to be stored at 2-8 degrees Celsius. But since no vaccine candidate has been approved by the WHO as yet, its administrative protocol and logistics are still unknown to us. If it turns out that the approved vaccines need to be stored at much colder temperature, we will have no alternative but to upgrade the system.
Therefore, it will be a mistake to completely rely on our existing cold chain for storing, transporting and distributing the Covid-19 vaccines. Although the government should have thought about it in advance, there's still time. The government should now focus on upgrading the existing vaccine cold chain network so that after procurement, vaccines can be transported to the remote parts of the country and administered rapidly at the right temperature.

 For all latest news, follow The Daily Star's Google News channel.
For all latest news, follow The Daily Star's Google News channel. 



Comments